What is Crenolanib?
Crenolanib is an investigational drug in advanced clinical development for patients with acute myeloid leukemia (AML) that contains a defect in the gene called FLT3 (Fms-like tyrosine kinase 3) and for patients with gastrointestinal stromal tumors (GIST) that contain defects in the gene called PDGFRA (platelet derived growth factor a). Crenolanib is also a potent and specific inhibitor of several cellular receptors closely related to FLT3 and PDGFRA which can also be dysregulated in various cancers.
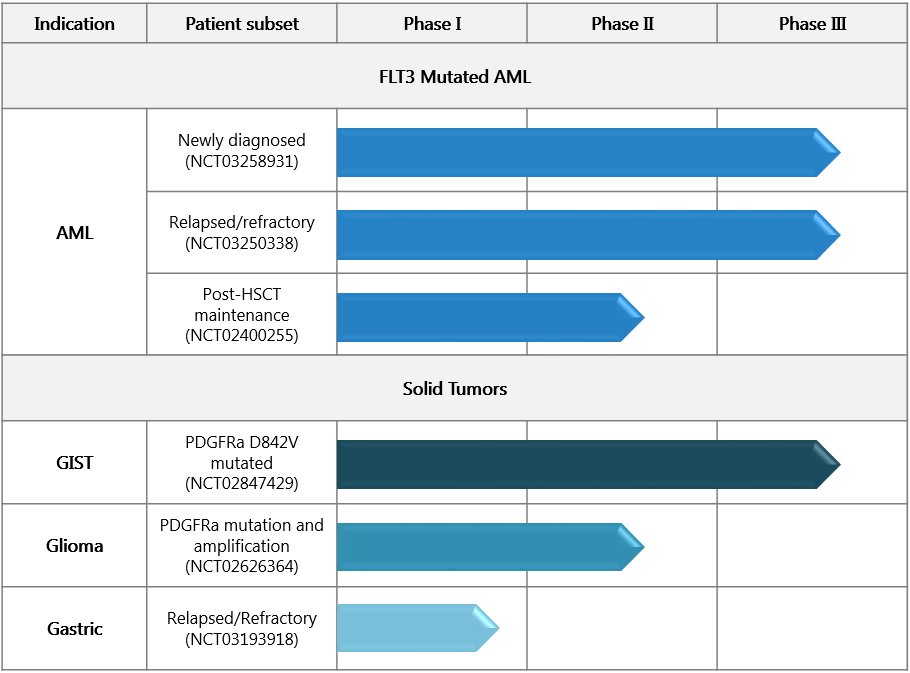
Crenolanib has shown promise in FLT3 mutant AML when combined with standard chemotherapy for the treatment of newly diagnosed patients, and in combination with salvage chemotherapy in relapsed or refractory patients. Additionally, crenolanib monotherapy has demonstrated encouraging activity in PDGFRA mutant GIST.
Crenolanib is also being evaluated in phase I-II trials in advanced glioma and gastric cancer.
FLT3 Summary
One of the most common mutations in AML is in the gene FLT3 (Fms-like tyrosine kinase 3), occurring in approximately 37% of all AML patients. Multiple FLT3 mutations have been identified and are a poor prognostic marker in AML. Traditional chemotherapy is often less effective in treating cancer cells harboring FLT3 mutations. Adding a FLT3 inhibitor to standard chemotherapy has been shown to produce longer lasting benefits compared to chemotherapy alone.
PDGFR Summary
Aberrations in PDGFR have an incidence of about 30% in cancer, including mutations, deletions, and amplifications. Tumor types for which PDGFR is altered in at least 10% of cases includes GIST, glioblastoma, lung, melanoma, bladder, prostate, colorectal and ovarian cancers.

